Applications
Possible applications of gene drive organisms
Gene drives present many novel potential applications. Current research is focused on three areas:
- the control of disease vectors
- the removal of invasive species from sensitive ecosystems
- the control of so-called pests in agriculture.
Gene drives to eliminate disease vectors
Infectious diseases such as malaria, dengue fever and borreliosis are transmitted to humans by mosquitoes or ticks. Combating these vectors has long been one of the most important disease prevention measures.
Gene drives are designed to take these efforts to a new level.
The malaria pathogen is spread exclusively by Anopheles mosquitoes. A concerted global programme of malaria control using mosquito nets, insecticides and medicines has helped to reduce the disease in many regions of the world and cut the number of deaths by about half between 2000 and 2015.1 In 2016, WHO identified 21 countries with the potential to achieve zero native malaria cases by 2020.
Of these, 37 countries are already certified2 as malaria-free most recently Sri Lanka (2016), Paraguay (2018) and Algeria (2019). China, Malaysia, El Salvador and Iran are also well on the way to achieving the three-year malaria-free status required for certification. Other factors for successfully combating the disease are, above all, strong political will, a functioning health system, good training of medical staff, national programmes for education and prevention measures, malaria screening, rapid and correct diagnosis, treatment and rapid responses to outbreaks of the disease.3
However, there are still 87 countries where such measures have not been sufficiently implemented. In 2017, more than 200 million people still contracted malaria and more than 400,000 people died of it.4 The poor regions of Africa are the hardest hit, with very high mortality rates among children under five years of age in particular. Gene drives are intended to remedy this situation by massively reducing the number of Anopheles mosquitoes in Africa and preventing the transmission of malaria.
The international research consortium Target Malaria plays a leading role in the development of such gene drives. The consortium has a budget of around 100 million US dollars, largely funded by the Bill & Melinda Gates Foundation5 and the Open Philanthropy Project.6
Target Malaria pursues two different approaches to controlling mosquito populations. One aims to produce sterile female Anopheles mosquitoes and spread this trait in the wild population using a CRISPR gene drive. In 2018, experiments in large cages showed that this approach basically works: the gene drive caused the population to collapse after about ten generations.7
Target Malaria’s second approach is to manipulate the mosquitoes’ gender distribution. A variant of the gene drive called ‚X-Shredder‘ ensures that predominantly male mosquitoes are born. This gene drive variant has also already been tested on mosquitoes in cages. Model calculations predict that releasing gene drive mosquitoes into the wild several times would reduce natural populations by up to 60 per cent, but probably not eradicate them.8
While Target Malaria aims to reduce the number of mosquitoes, gene drive developers at the University of California, San Diego are taking a different approach. With a multi-million dollar grant from the Indian Tata Foundation9, they are looking for a way to create resistance in Anopheles mosquitoes that kills the malaria pathogen and prevents people from becoming infected.10 In initial lab experiments, however, such gene drive organisms have proven to have only limited viability.11
The plans of Target Malaria, on the other hand, have already reached the stage where the first model projects have been launched in Burkina Faso, Mali, Ghana and Uganda. In July 2019, Target Malaria in Burkina Faso carried out the first tests, which are regarded as a preliminary stage for the future release of gene drive mosquitoes.12 Target Malaria plans to release the first gene drive mosquitoes as early as 2024. Although Target Malaria points out that the local population is involved in the decision making process, previous experiments have led to national and international protests. 13/14
In temperate climate zones, the use of gene drives against the infectious disease Lyme disease is being considered. In the USA, Lyme disease has recently become very widespread and affects about 300,000 people annually.15 In Germany, the number of new cases is estimated to be about 100,000 per year.16
This disease is caused by Borrelia bacteria, which often infest wild mice and are transmitted from ticks to humans. If the infection is not detected in time, a chronic, difficult to treat disease can develop.
On two islands in the northeast of the USA, a project has been started to interrupt the transmission of the disease using genetic engineering.17 The tick disease vectors are not the target of genetic manipulation, but the native white-footed mice, which are the most important reservoir of Borrelia in these regions. They seek to intervene in the immune system of the mice to make them resistant to infection and interrupt the Borrelia transmission chain.
After a public survey on the islands of Nantucket and Martha’s Vineyard in Massachusetts, USA, a majority was found to be in favour of preparing field experiments, but rejected the use of gene drives. The plan now is to release genetically modified mice on a massive scale to mate with their natural conspecifics and crossbreed Borrelia resistance into the population. If, however, experiments on larger landmasses are planned in the future, the use of gene drive mice would again be up for discussion.
However, the use of gene drive in combatting Lyme disease, since infection can be prevented by simple means: wearing suitable clothing, applying anti-tick agents and regularly scanning the body. A vaccine was even available for a short time, but disappeared from the market due to lack of interest. The use of genetic engineering methods is therefore not due to a lack of alternatives, but because the alternatives are generally perceived as annoying and disturbing.
Using gene drives against invasive species
Humans have brought numerous animal species to foreign islands and continents, where they have become a serious threat to the native flora and fauna. For example, imported rats and mice cause great problems, decimating smaller animals and interrupting the breeding of native birds. Conventional measures such as hunting, traps or poisoned bait have sometimes succeeded in eliminating invasive species from small islands, but on larger landmasses, these measures are reaching their limits. Gene drives offer a possible alternative.
This approach is being pursued by the Genetic Biocontrol of Invasive Rodents (GBIRd) project, which is supported by seven universities, authorities and non-governmental organisations from the USA and Australia. GBIRd aims to clarify whether invasive mice can be eradicated by gene drives and under what conditions this intervention would be acceptable. The majority of the project is funded by 6.4 million US dollars from the US Defence Advanced Research Projects Agency (DARPA).18
One of the most active members of GBIRd is the small conservation organisation, Island Conservation. For 25 years, it has been dedicated to the protection of seabirds and has, according to its own information, already freed 63 islands from rodents. So far, this has been done using conventional methods, but for further progress, Island Conservation believes the use of gene drives is necessary.
The first steps in this direction were taken at the University of California, San Diego, USA, where gene drives for mice were developed for the first time in 2019.19 However, the developers encountered an unexpected phenomenon: CRISPR/Cas9 was able to cut the DNA strand in all experimental animals, but only in females did the repair mechanism that actively disseminates the new DNA segments in the genome begin. The gene drive was therefore successful in only one of the two sexes, and even there it only achieved an efficiency of about 70 per cent. Gene drives of this kind are probably not suitable for manipulating free-living populations.
Until recently, New Zealand also showed interest in using gene drives. The unique flora and fauna of this country suffers great damage from rats, stoats and the Australian fox kusu. With the Predator Free 2050 programme, the New Zealand government is pursuing the ambitious goal of eradicating all invasive predators by the year 2050. This has already been successful on more than 100 smaller islands, but on the main islands, eradication by conventional methods alone is scarcely achievable.
In this context, two gene drive developers published an article in 2017 warning against premature release and the use of gene drive organisms in nature conservation.20 After a change of government in the same year, New Zealand has become more cautious. Before Predator Free returns to the political forefront, numerous technical, social, ethical considerations and regulatory hurdles need to be researched and overcome.21
Gene Drives in Agriculture
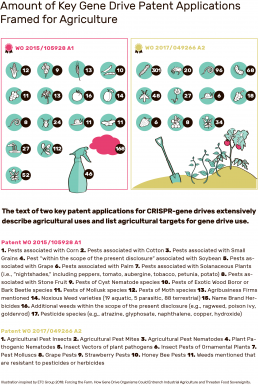
In the long term, agriculture could become the most important field of application for gene drives – a fact that has so far hardly been noticed by the public. Patents on CRISPR-based gene drives list hundreds of animals and plants whose containment or eradication could increase agricultural yields. However, there are still some hurdles to be overcome.
At least six patents on gene drives refer to concrete applications in agriculture. The focus is on the control of pests and weeds and the withdrawal of herbicide resistance. Two key applications come from leading developers of the CRISPR-based gene drives: the research groups around Kevin Esvelt22 and Ethan Bier23. Bruce Hay’s group24 has also made numerous claims about their technology in a patent application. In most cases, the claims are general, but a patent already contains detailed objectives and methods that enable commercial use.
However, the commercialisation of gene drives faces a fundamental problem: their distribution cannot yet be limited in terms of either time or space. Individual releases could result in the spread of the GDO into transboundary ecosystems for decades. The classic business model of agricultural companies, which is based on the continuous sale of products, would be difficult to apply under these conditions.
In theory, the use or gene drives appears commercially interesting in only two scenarios. A gene drive could eliminate natural resistance that wild plants have developed against common herbicides – an agricultural company could then benefit from the increased sales of the herbicide because they would become usable again. Or, large farming associations could finance the development of gene drive, which would benefit all members.
Examples of applications in agriculture
Gene drives would be of conceivable relevance for issues facing the cultivation of almost any crop, the husbandry of numerous farm animals or for addressing so-called pests.
In three cases, there are already concrete plans:
The spotted wing Drosophila (Drosophila suzukii), originally native to Southeast Asia, has spread worldwide and is causing considerable crop losses for numerous varieties of fruit. It lays its eggs in almost ripe, undamaged fruit with thin skins. In 2008, the cherry vinegar fly reached California and caused damage of over 38 million US dollars in cherry plantations the following year alone. According to calculations, these losses could rise to over 500 million US dollars annually in the western USA.25 Since 2011, it has also appeared in Germany and endangers the harvest of cherries, grapes, raspberries, blackberries and strawberries.26
The California Cherry Growers’ Association, the California Cherry Board, began funding research for a gene drive in 2013 with annual funding of 100,000 US dollars.27 A group of researchers at the University of San Diego, USA, developed a so-called Medea Drive. This can control wild populations in various ways: the offspring of the flies are either non-viable or sterile. These effects can affect one sex or both. In initial lab experiments, a large number of modified flies were necessary to establish the Medea Drive in the population. In addition, many fly populations in the wild exhibit natural resistance, which would probably strongly impede the spread of the Medea Drive. The researchers therefore assume that a very large number of modified cherry vinegar flies would have to be released in order to keep the Medea Drive in the population for several years. Outdoor tests are not yet planned.28
The patent registered on this Medea Drive29 in 2017 also covers other species of tropical fruit flies and mosquitoes of the genera Anopheles and Aedes, which transmit malaria and numerous viral diseases.
Other potential target organisms for gene drives are plant lice. In 2005, bacteria that attack citrus trees and make their fruit inedible were detected for the first time in the USA. The bacteria are spread by Asian plant lice that infest citrus trees. When they suck the sap of plants, they take up the bacteria and can then infect other trees. Within three years, the disease, which goes by the name of Huanglongbing, has spread over most of Florida’s growing areas, with citrus fruit production falling by 70 per cent.30 Europe has so far been spared the disease, but its further spread cannot be ruled out.31
Citrus fruit producers in California are considering the use of gene drives to protect their growing areas.32 One possibility would be the release of gene drive plant lice, which cannot transmit the bacteria. A research project on this was completed in 2017 and has identified a number of genes that could prevent transmission. However, a gene drive has not yet been established.33
The New World screw-worm fly (Cochliomyia hominivorax) is found mainly on the American continent and lays its eggs near body orifices or open wounds of mammals and birds. The hatching larvae eat deep into the tissue of the infected animals and cause severe inflammation. The New World screwworm fly also infests farm animals such as cows, sheep and goats, which can die from the inflammation without veterinary treatment.34
The screwworm fly was eradicated in the 1960s on the mainland of the USA and in Central America by the release of sterile male flies. To prevent new immigration from South America, a protection zone was established in Panama, but its maintenance is very expensive. Scientists at the University of North Carolina in the USA have therefore proposed the use of gene drives.35 In addition, the extermination of the screwworm fly in South America would also be conceivable through the use of gene drives.
In 2019, an international group of researchers was able to switch off a gene in the fly for the first time with CRISPR/Cas9. This resulted in females that exhibit male sexual characteristics and are presumably sterile.36 This intervention is a first step towards the development of a gene drive that would aim to completely eradicate the screwworm fly.
In theory, gene drives could also be used in plants. The U.S. National Academies of Science identified the foxtail plant Amaranthus palmeri, which has developed into a resistant ‚superweed‘38 in the USA beginning in the 1990s due to the excessive use of herbicides such as glyphosate. Amaranthus palmeri37 is a dioecious plant, which produce either male or female flowers. Researchers recently identified a gene that controls the formation of female flowers.39 Should it become possible to switch off this gene by means of a gene drive, only male plants could be formed and make natural reproduction impossible.
Another theoretical possibility would be the withdrawal of resistance to common pesticides, which has developed in dozens of plant species and which poses major problems for industrial agriculture. Behind these resistances are genetic changes that are often well researched and could theoretically be reversed by a gene drive.40
Before gene drives can be used in plants, however, a number of technical hurdles still have to be overcome. For example, CRISPR/Cas9 works only very inefficiently under these conditions, since plant cells usually repair their genome with error-prone mechanisms.41 Moreover, many plants have significantly longer generational cycles than insects: the effect of a gene drive would therefore only be felt after many years. And finally, the seeds of some plants can survive in the soil for years and significantly delay the breakthrough of the gene drive.42 The utilisation of gene drives in plants is not yet possible with the current state of knowledge.
This figure illustrates the areas in which gene drive organisms are being developed or considered for agricultural application.
Gene drives to eradicate rats, mice, cockroaches and moths that infest grain silos.
Gene drives to eradicate the New World screwworm fly, which lays its eggs in the body cavities and wounds of cows and other farm animals.
Gene drives to eradicate the Cherry Vinegar Fly, which lays its eggs in ripe fruit, such as cherries.
Gene drives to eradicate leaf fleas, which spread Cirtrus Greening Disease (Huanglongbing) in citrus fruits.
Gene drives to eradicate nematodes that cause plant diseases.
Gene drives to decimate the cabbage moth.
Gene Drives as biological weapons
The release of gene drive organisms could have widespread and long-lasting negative effects on ecosystems and societies. For this reason alone, gene drive organisms could be misused as biological weapons against plants, animals and humans. The direct development of gene drive organisms for hostile purposes is also conceivable.
One way in which gene drives could be used as biological weapons would be to use them to eradicate important beneficial insects for agriculture in a particular region – similarly to how malaria-transmitting mosquitoes are to be decimated. However, as long as gene drives and their harmful effects cannot be localized in time or space, there are few convincing scenarios for governmental gene drive weapon programs.44
There are many grey areas surrounding unexpected negative effects of gene drive organisms in nature, the misuse of gene drives for hostile purposes and the deliberate development of gene drives for hostile purposes. The effect of a gene drive in a certain region can be assessed as positive there, while the consequences in other affected regions could be undesirable or strongly negative. The Meetings of Experts (MX) of the UN Biological Weapons Convention have been critically observing and discussing the issue for years. 45
There are many scenarios in which the use of gene drives could lead to conflict. Conflict can also arise over a lack of public (or international) consensus on a release in one’s own or a neighbouring country, or because it causes damage, e.g. loss of crops, loss of biodiversity or unintended health, social or economic effects, for which there is no adequate compensation. The mere unintentional presence of a GDO in a country that has not agreed to a release could lead to interstate conflicts or diplomatic crises. 46
The US Defence Advanced Research Projects Agency (DARPA) is one of the largest donors to gene drive research and is financially involved in almost every gene drive research project.47 The DARPA research program, entitled Safe Genes, aims to control, limit, or reverse the effects of released gene drive organisms in the environment.48
Sources
- World Health Organization, and the United Nations Children’s Fund (2015). Achieving the malaria MDG target: reversing the incidence of malaria 2000–2015. Online: https://www.who.int/malaria/publications/atoz/9789241509442/en/ [letzter Zugriff: 09.03.2020]
- World Health Organization Website (2019). World Health Organisation. Countries and territories certified malaria-free by WHO. Online: https://www.who.int/malaria/areas/elimination/malaria-free-countries/en/ [letzter Zugriff: 09.03.2020]
- Global Malaria Programme, World Health Organization (2019). The E-2020 initiative of 21 Malaria-eliminating countries. 2019 progress report. Online: https://apps.who.int/iris/bitstream/handle/10665/325304/WHO-CDS-GMP-2019.07-eng.pdf?ua=1 [letzter Zugriff: 09.03.2020]
- World Health Organization (2018). World Malaria Report 2018. World Health Organization. Online: https://www.who.int/malaria/publications/world-malaria-report-2018/en/ [letzter Zugriff: 09.03.2020]
- Regalado A, MIT Technology Review Website (2016). MIT Technology Review; c2020. Bill Gates Doubles His Bet on Wiping Out Mosquitoes with Gene Editing. Online: https://www.technologyreview.com/s/602304/bill-gates-doubles-his-bet-on-wiping-out-mosquitoes-with-gene-editing/ [letzter Zugriff: 09.03.2020]
- Dunning H, Imperial College London Website (2017). London: Imperial College London; c2020. Malaria elimination project wins $17.5m funding boost. Online: https://www.imperial.ac.uk/news/179689/malaria-elimination-project-wins-175m-funding/ [letzter Zugriff: 09.03.2020]
- Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A (2018). A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol 36:1062.
- Facchinelli L, North AR, Collins CM, Menichelli M, Persampieri T, Bucci A, Spaccapelo R, Crisanti A, Benedict MQ (2019). Large-cage assessment of a transgenic sex-ratio distortion strain on populations of an African malaria vector. Parasit Vectors 12:70.
- Philanthropy News Digest Website (2016). c2020. Tata Trust Awards $70 Million to UC San Diego for Genetics Institute. Online: https://philanthropynewsdigest.org/news/tata-trusts-awards-70-million-to-uc-san-diego-for-genetics-institute [letzter Zugriff: 09.03.2020]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, James AA (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A. 8:E6736 .
- Pham TB, Phong CH, Bennett JB, Hwang K, Jasinskiene N, Parker K, Stillinger D, Marshall JM, Carballar-Lejarazú R, James AA (2019). Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet 15:e1008440.
- Diabate A, Target Malaria Website. (2019). Target Malaria; 2020. Target Malaria proceeded with a small-scale release of genetically modified sterile male mosquitoes in Bana, a village in Burkina Faso. Online: https://targetmalaria.org/target-malaria-proceeded-with-a-small-scale-release-of-genetically-modified-sterile-male-mosquitoes-in-bana-a-village-in-burkina-faso/ [letzter Zugriff: 09.03.2020]
- Wallace H, Li Ching L, Mayet M, African Center for Biodiversity Website (2018). African Center for Biodiversity. Release of risky GM mosquitoes in Burkina Faso highly unethical. Online: https://www.acbio.org.za/en/release-risky-gm-mosquitoes-burkina-faso-highly-unethical [letzter Zugriff: 09.03.2020]
- Fuhr L, Klima der Gerechtigkeit Website (2018). Heinrich-Böll-Stiftung e.V. Burkina Faso’s Mosquito Controversy: Consent, awareness and risk assessment in Target Malaria’s gene drive project. Online: https://klima-der-gerechtigkeit.de/2018/11/20/burkina-fasos-mosquito-controversy-consent-awareness-and-risk-assessment-in-target-malarias-gene-drive-project/ [letzter Zugriff: 09.03.2020]
- Centers for Disease Control and Prevention Website (2019). U.S. Department of Health & Human Services. Lyme Disease – Data and Surveillance. Online: https://www.cdc.gov/lyme/datasurveillance/index.html [letzter Zugriff: 09.03.2020]
- Robert Koch-Institut Website (2018). Robert Koch-Institut. Borreliose – Antworten auf häufig gestellte Fragen zu Borreliose. Online: https://www.rki.de/SharedDocs/FAQ/Borreliose/Borreliose.html [letzter Zugriff: 09.03.2020]
- Buchthal J, Evans SW, Lunshof J, Telford SR 3rd, Esvelt KM (2019). Mice Against Ticks: an experimental community-guided effort to prevent tick-borne disease by altering the shared environment. Philos Trans R Soc Lond B Biol Sci 374:20180105.
- Neslen A, The Guardian Website (2017). Guardian News & Media Limited; c2020. US military agency invests $100m in genetic extinction technologies. Online: https://www.theguardian.com/science/2017/dec/04/us-military-agency-invests-100m-in-genetic-extinction-technologies [letzter Zugriff: 09.03.2020]
- Grunwald HA, Gantz VM, Poplawski G, Xu XS, Bier E, Cooper KL (2019). Super-Mendelian inheritance mediated by CRISPR-Cas9 in the female mouse germline. Nature 566:105.
- Esvelt KM, Gemmell NJ (2017). Conservation demands safe gene drive. PLoS Biol. 15:e2003850.
- Murphy EC, Russel JC, Broome KG, Ryan GJ, Dowding JE (2019). Conserving New Zealand’s native fauna: a review of tools being developed for the Predator Free 2050 programme. Journal of Ornithology 160:883.
- Esvelt KM, Smidler AL (2015). RNA-guided gene drives. Patent No. WO/2015/105928.
- Bier E, Gantz V (2016). Method for autocatalytic genome editing and neutralizing autocatalytic genome editing. Patent No. WO/2016/073559.
- Hay BA, Oberhofer G, Ivy TW (2018). DNA sequence modification-based gene drive. Patent No. WO 2018/204722A1.
- Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O’Neal SD, Zalom FG (2011). Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. Journal of Integrated Pest Management 2: G1.
- Bundesministerium für Ernährung und Landwirtschaft Website (2019). Bundesministerium für Ernährung und Landwirtschaft. Kirschessigfliege: Herkunft und Bedeutung. Online: https://www.bmel.de/DE/Landwirtschaft/Pflanzenbau/Pflanzenschutz/_Texte/Kirschessigfliege_Management.html [letzter Zugriff: 11.03.2020]
- Regalado A, MIT Technology Review Website (2017). MIT Technology Review; c2020. Farmers Seek to Deploy Powerful Gene Drive. Online: https://www.technologyreview.com/s/609619/farmers-seek-to-deploy-powerful-gene-drive/ [letzter Zugriff: 10.03.2020]
- Buchman A, Marshall JM, Ostrovski D, Yang T, Akbari OS (2018). Synthetically engineered Medea gene drive system in the worldwide crop pest Drosophila suzukii. PNAS 115:4725.
- Akbari OS, Buchman A (2017). Use of medea elements for biocontrol of D. suzukii populations. Patent No. WO 2017/132207.
- Citrus Research Board (2017). CLB HLB external scientific review – Final report. 2017 Aug 14-18; Davis, California, USA. Online: http://citrusresearch.org/wp-content/uploads/HLB-External-Review_FINAL-Report.pdf [letzter Zugriff: 11.03.2020]
- Pérez-Rodríguez J, Krüger K, Pérez-Hedo M, Ruíz-Rivero O, Urbaneja A, Tena A (2019). Classical biological control of the African citrus psyllid Trioza erytreae, a major threat to the European citrus industry. Sci Rep 9:9440.
- Citrus Research Board (2017). CLB HLB external scientific review – Final report. 2017 Aug 14-18; Davis, California, USA. Online: http://citrusresearch.org/wp-content/uploads/HLB-External-Review_FINAL-Report.pdf [letzter Zugriff: 11.03.2020]
- United States Department of Agriculture Website, Citrus Research and Development (2017). United States Department of Agriculture. Source: Citrus Research & Development Foundation (CRDF) submitted to Rear and Release Psyllids as Biological Control Agents – An Economical and Feasible Mid-Term Solution for Huanglongbing (HLB) Disease. Online: https://reeis.usda.gov/web/crisprojectpages/0230893-rear-and-release-psyllids-as-biological-control-agents–an-economical-and-feasible-mid-term-solution-for-huanglongbing-hlb-disease.html [letzter Zugriff: 11.03.2020]
- Scott MJ, Concha C, Welch JB, Philips PL, Skoda SR (2017). Review of research advances in the screwworm eradication program over the past 25 years. Entomologia Experimentalis et Applicata 164:226.
- Paulo DF, Williamson ME, Arp AP, Li F, Sagel A, Skoda SR, Sanchez-Gallego J, Vasquez M, Quintero G, Pérez de León AA, Belikoff EJ, Azeredo-Espin AML, McMillan WO, Concha C, Scott MJ (2019). Specific Gene Disruption in the Major Livestock Pests Cochliomyia hominivorax and Lucilia cuprina Using CRISPR/Cas9. G3:Genes, Genomes, Genetics 9:3045.
- Ebenda
- National Academies of Sciences, Engineering, and Medicine (2016). Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. Washington, DC: The National Academies Press. https://doi.org/10.17226/23405.
- Webster TM, Nichols RL (2012). Changes in the prevalence of weed species in the major agronomic crops of the Southern United States: 1994/1995 to 2008/2009. Weed Science 60:145.
- Montgomery JS, Sadeque A, Giacomini DA, Brown JB, Tranel PJ (2019). Sex-specific markers for waterhemp (Amaranthus tuberculatus) and Palmer amaranth (Amaranthus palmeri). Weed Science 67:412.
- Neve P (2018). Gene drive systems: do they have a place in agricultural weed management? Pest Manag Sci 74:2671.
- Hahn F, Eisenhut M, Mantegazza O, Weber APM (2018). Homology-Directed Repair of a Defective Glabrous Gene in Arabidopsis With Cas9-Based Gene Targeting. Frontiers in Plant Science 9:424.
- Barrett LG, Legros M, Kumaran N, Glassop D, Raghu S, Gardiner DM (2019). Gene drives in plants: opportunities and challenges for weed control and engineered resilience. Proc Biol Sci. 286:20191515.
- National Academies of Sciences, Engineering, and Medicine (2016). Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. Washington, DC: The National Academies Press. https://doi.org/10.17226/23405. p. 161.
- Jeremias G (2019). Governing the Conflict Potential of Novel Environmental Biotechnologies (NEBs). BWC Meeting of State Parties; 2019 Dec 3. Online: https://www.unog.ch/80256EDD006B8954/(httpAssets)/FABC68A345728CFFC12584C7006218F4/$file/Conflict+potentials+from+Gene+Drives2.pdf [letzter Zugriff: 11.03.2020]
- Chair of the Meeting of Experts on Review of Developments in the Field of Science and Technology Related to the Convention (2018). Meeting of Experts on Review of Developments in the Field of Science and Technology Related to the Convention: Reflections and proposals for possible outcomes. 2018 Meeting of the States Parties to the Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on Their Destruction; 2018 Dec 4-7; Geneva, Switzerland. Online: https://www.unog.ch/80256EDD006B8954/(httpAssets)/327ACB8D34AFD3C8C12583930032B711/$file/CRP_3.pdf [letzter Zugriff: 11.03.2020]
- Jeremias G (2019). Governing the Conflict Potential of Novel Environmental Biotechnologies (NEBs). BWC Meeting of State Parties; 2019 Dec 3. Online: https://www.unog.ch/80256EDD006B8954/(httpAssets)/FABC68A345728CFFC12584C7006218F4/$file/Conflict+potentials+from+Gene+Drives2.pdf [letzter Zugriff: 11.03.2020]
- Gene Drive Files Website (2017). Gene Drive Files. Gene Drive Files Expose Leading Role of US Military in Gene Drive Development. Online:http://genedrivefiles.synbiowatch.org/2017/12/01/us-military-gene-drive-development/ [letzter Zugriff: 11.03.2020] und: Gene Drive Files. AS notes on DARPA Safe Genes rollout San Diego May 2 2017. Online: /http://genedrivefiles.synbiowatch.org/as-notes-on-darpa-safe-genes-rollout-san-diego-may-2-2017/ [letzter Zugriff: 11.03.2020]
- Defense Advanced Research Projects Agency Website (2019). Defense Advanced Research Projects Agency. Safe Genes Tool Kit Takes Shape – Successes in first two years of Safe Genes program establish technological foundations and ground truth in support of DARPA’s emerging, adaptable resources for secure genome editing research. Online: https://www.darpa.mil/news-events/2019-10-15 [letzter Zugriff: 11.03.2020]