Ecological risks
As gene drives are in an early stage of development, the discussion about their possible consequences and risks is still largely speculative. However, numerous critical points are already emerging that need to be considered before a possible release.
Uncontrollability
Once released into the wild, a gene drive organism actively propagates in free-living populations and can rapidly spread over large distances. The unmanageable diversity of the natural habitats and ecosystems affected will make it massively more difficult to predict and control possible risks.
The US Academy of Sciences therefore recommended in 2016 that gene drive organisms be tested first on small and remote islands.1However, model calculations show that this would hardly suffice: Individual GDOs can reach other regions by water, wind or unintentional transport and further spread the gene drive.2 Moreover, the GDO could be deliberately propagated, as a precedent case from 1997 shows: In New Zealand, farmers arbitrarily released a dangerous virus to control a rampant rabbit plague.3
A group of researchers led by gene drive developer Kevin Esvelt of the Massachusetts Institute of Technology (MIT) in Boston, USA, is working on a gene drive variant that would be limited in its spatial spread. This gene drive is called Daisy Chain Drive.4 However, this gene drive variant only exists in theory. Some researchers have expressed serious doubts about its feasibility.5
Irreversibility
A gene drive causes a permanent genetic modification of the genetic material, which is passed on to all subsequent generations. Even if a gene drive encounters resistance and no longer spreads via its own mechanism of action, these changes are still inherited according to Mendel’s laws and continue to persist in the genome of the population for a long time. Only if the deactivated gene drive severely impairs the survival of the individuals would the mechanisms of natural selection, which could eliminate the changes in the natural populations, take effect.
As early as 2014, a discussion began about the need for a so-called reversal drive, which would reverse the changes of a gene drive in the manipulated populations. In principle, this is a modified version of the original gene drive, but it overwrites the genetic manipulations and prevents their further spread. However, even such a reversal drive could not restore the original genetic state of the population, but only introduce further genetic modifications into the genome of these populations. According to current knowledge, every release of a gene drive carries the risk that the genetic material of a natural population will be irreversibly and uncontrollably altered.6
Outcrossing across species boundaries
Gene drives are tailored to the genome of a single species, but in many cases outcrossing across species boundaries would likely be impossible to prevent.
The malaria-transmitting mosquito Anopheles gambiae, for example, is part of a complex of six different species that are genetically very similar and can produce fertile offspring.7 A gene drive developed by Target Malaria aims at disrupting the gene Doublesex, which has undergone only minor changes during the evolution of the mosquito species. This approach could drive all six related mosquito species to the brink of extinction, although at least one is not a carrier of malaria.8
A similar risk exists in fruit flies of the genus Drosophila, which play a central role in the development and application of gene drives. It has been known for over 90 years that different Drosophila species can crossbreed and produce fertile offspring.9Thousands of other animal and plant species form natural hybrids, so that the use of gene drives would not be limited to one species, but could also be transferred to their close relatives.
Unpredictable effects of CRISPR/Cas9
Many gene drives use the genetic engineering tool CRISPR/Cas9 to create a double-strand break at defined points in the genome. However, this tool does not work without errors. CRISPR/Cas9 can change the activity of the target gene in unpredictable ways, increase the mutation rate in the genome, lead to unexpected mutations or be disrupted in its function by emerging resistance.
The genetic modifications often affect not only the target area, but often other areas of the genome as well (off-target effects).11One reason for this may be that there are more sequences in the genome of wild populations of an organism to which CRISPR/Cas9 can dock than the computer programs used for this purpose were able to identify in the laboratory. Gene drives can therefore lead to the development of organisms with unpredictable characteristics.12
Resistance
CRISPR-based gene drives search for a clearly defined DNA sequence at which they should cut the genome. Even single mutations in this sequence can therefore make the target invisible to them. The organism thus becomes resistant to the gene drive. Such resistance can arise when CRISPR/Cas9 itself generates mutations that destroy the target sequence. However, they could also occur naturally, especially in populations with high genetic diversity.
If a gene drive encounters resistance, it will stop at this point and only alter part of the population. However, whether it disappears completely depends on the number of individuals that have already been altered and the disadvantages that the gene drive has for their survival. It is therefore quite possible that the gene drive will continue to survive for a long time in an animal species despite resistance.
Unpredictable effects on ecosystems
Every living creature, even if it appears dangerous or harmful to humans, fulfils important tasks in its habitat. The extermination or even manipulation of a species will therefore have consequences for the entire ecosystem.
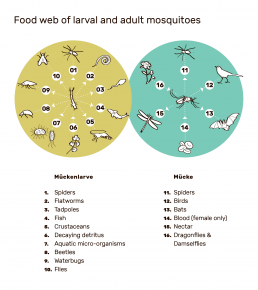
This can be clearly illustrated using the example of mosquitoes. In the course of their life cycle, they form important food sources for various animals. Mosquito larvae living in water are, for example, a food source for water bugs, beetles, flies, spiders, flatworms, tadpoles, fish and crustaceans. About 95 per cent of larvae of the African malaria mosquito Anopheles gambiae are believed to consumed before they reach adulthood.13
Adult mosquitoes are also an important source of food and are eaten by dragonflies, spiders, bats and birds, among others. In the Camargue, a nature reserve in southern France, the decimation of mosquitoes with a biological pesticide has also led to a reduction in the number and diversity of birds and dragonflies.14
A role in plant pollination cannot be ruled out either, since adult mosquitoes also feed on nectar.15 The role of mosquitoes in their closely-knit ecosystem has barely been investigated to date, so the consequences of possible extinction are not predictable.
- National Academies of Science, Engineering and Medicine (2016). Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values. Washington, DC: The National Academies Press. https://doi.org/10.17226/23405.
- Noble C, Adlam B, Church GM, Esvelt KM, Nowak MA (2017). Current CRISPR gene drive systems are likely to be highly invasive in wild populations. Elife 7:e33423.
- O’Hara P (2006). The illegal introduction of rabbit haemorrhagic disease virus in New Zealand. Rev Sci Tech 25:119.
- Noble C, Min J, Olejarz J, Buchthal J, Chavez A, Smidler AL, DeBenedictis EA, Church GM, Nowak MA, Esvelt KM (2019). Daisy-chain gene drives for the alteration of local populations. Proc Natl Acad Sci USA 116:8275.
- ebenda
- Esvelt KM, Smidler AL, Catteruccia F, Church GM (2014). Concerning RNA-guided gene drives for the alteration of wild populations. Elife 17:e03401.
- Coluzzi M, Sabatini A, Petrarca V, Di Deco MA (1979). Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R SocTrop Med Hyg 73:483.
- Critical Scientists Switzerland (CSS), European Network of Scientists for Social and Environmental Responsibility (ENSSER), Vereinigung Deutscher Wissenschaftler (VDW) (2019). Gene Drives. A report on their science, applications, social aspects, ethics and regulations. p. 101. Online: https://genedrives.ch/report [letzter Zugriff: 11.03.2020]
- Barbash DA (2010). Ninety years of Drosophila melanogaster hybrids. Genetics 186:1.
- Kosicki M, Tomberg K, Bradley A (2018). Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol 36:765.
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013). High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822.
- Lindholm AK, Dyer KA, Firman RC, Fishman L, Forstmeier W, Holman L, Johannesson H, Knief U, Kokko H, Larracuente AM, Manser A, Montchamp-Moreau C, Petrosyan VG, Pomiankowski A, Presgraves DC, Safronova LD, Sutter A, Unckless RL, Verspoor RL, Wedell T (2016). The Ecology and Evolutionary Dynamics of Meiotic Drive. Trends Ecol Evol. 31:315-326.
- Collins CM, Bonds JAS, Quinlan MM, Mumford JD (2019). Effects of the removal or reduction in density of the malaria mosquito, Anopheles gambiae s.l., on interacting predators and competitors in local ecosystems. Med Vet Entomol 33:1.
- Jakob C, Poulin B (2016). Indirect effects of mosquito control using Bti on dragonflies and damselflies (Odonata) in the Camargue. Insect Conservation and Biodiversity 9:161.
- Foster WA (1995). Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol 40:443.