What are Gene Drive Organisms?
With the help of new genetic engineering techniques such as CRISPR/Cas9, so-called gene drives have been developed in recent years. They enable humans to spread new genes in the genome of wild animal populations. Furthermore Gene Drives force the inheritance of newly introduced genes to all offspring, even if this lowers the survival chances of the affected species. In the most extreme case, gene drive technology could drive an entire species to extinction or replace wild populations with genetically modified organisms.
Reproduction of the egoists
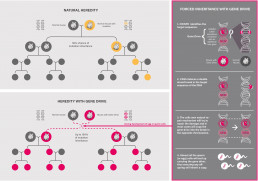
In nature, the process of evolution is slow: It takes many generations before inherited changes take hold. In sexual reproduction, genetic material recombines in each generation. New traits are in constant competition with older ones. However, only one of the two is passed on to the offspring. Which one is determined by chance. According to Mendel’s rules, the probability that a new trait will be passed on to the offspring is 50 percent. As a rule, a higher inheritance rate only occurs if the traits are associated with advantages for the survival of the species.
Jumping Genes
However, not all natural genetic traits follow these Mendelian rules of inheritance. In plants, animals and humans, there are genetic elements that copy themselves into other parts of the genome with the help of enzymes, spreading independently and thus increasing the frequency of their inheritance. They are now often referred to as naturally occurring gene drives. Examples are so-called jumping genes (transposons). ‘Selfish’ is the name given to these genes because they can spread throughout the genome without benefiting the species. During evolution, plants, animals and humans have found a way to deal with these genetic elements: Some gave rise to important functional, usually regulatory, units. In many other cases, mechanisms have been developed to silence the jumping genes in the genome (for more information, see info box).
Researching Gene Drive Organisms
Gene drives are based on a similar principle. In 2003, the British researcher Austin Burt formulated the idea that genes can spread rapidly if they overwrite competing variants. The natural evolutionary process then no longer works: humans can change the genetic material of free-living organisms and spread new characteristics to serve their purposes.¹
So-called ‘selfish’ genetic elements are found in the genome of almost all living beings. Their reproduction seems to be of no consequence in the short term. However, they play an important role within longer periods of evolution. They contribute to the emergence of new gene variants and may well facilitate adaptation to changing environmental conditions. Numerous protective mechanisms limit the uncontrolled multiplication of these elements in the genome and limit the damage to the living being.
Transposons
Transposons are among the most common ‘selfish’ elements.² They essentially consist of an enzyme that makes copies of the transposon and inserts them elsewhere in the genome. This is where the term ‘jumping genes’ comes from. They were originally discovered by Barbara McClintock, who was awarded the Nobel Prize in 1983.
Homing endonuclease genes
Another variety of selfish element is widespread in bacteria: homing endonuclease genes.³ These genes also consist of only a single enzyme and can insert themselves precisely into certain DNA sequences. They spread rapidly and can even cross between different species of bacteria. The so-called homing gene drives were designed according to their model.
From Gene Drives to Gene Drive Organisms
Gene drives, on the other hand, are selfish genetic elements created by humans. Their purpose goes far beyond their own propagation: they are intended to specifically exterminate living beings or to endow them with characteristics that serve human interests. Evolutionarily established mechanisms that protect the genetic material against selfish elements are likely to be ineffective. If gene drives become established in the genome, there is a risk of unforeseeable evolutionary changes in the manipulated organisms. Engineered or synthetic gene drives thus set in motion a ‘mutagenic chain reaction’,4 the consequences of which cannot be controlled.
Wolbachia bacteria
Some publications refer to Wolbachia bacteria as ‘natural’ gene drives. This is not quite correct: Wolbachia is a bacterial infection of insects that is heritable over generations.5 Wolbachia bacteria occur naturally in the cells of certain insects, such as fruit flies. They reduce the reproductive capacity of infected insects. Therefore, with the hope of combating dengue fever, mosquitoes of the species Aedes aegypti were infected with Wolbachia bacteria in the laboratory. It was found that certain Wolbachia bacteria can block the transmission of dengue fever to humans.6 Field trials with Wolbachia infected mosquitoes first took place in 2011 for testing purposes in Australia.7
Unlike synthetic gene drives, this approach does not use genetic engineering. This means that the risks of genetic side effects associated with genetic engineering through crossbreeding and interaction with wild populations are not relevant in Wolbachia interventions.
Natural Inheritance – Forced inheritance with Gene Drives
Gene Drive Organisms in the wild
Until now, all experiments with genetically engineered gene drives have taken place exclusively in the laboratory or in closed containers. But gene drives are intended for use in the wild. They are intended to introduce new genes into the genome of wild populations, even if these reduce the chances of survival of the species concerned. The goal of their use in the wild may be to replace the entire wild population with genetically modified gene drives organisms or to decimate them. In the most extreme case, deployment could drive the entire species to extinction.
The first field trials with gene drive mosquitoes could be carried out in Burkina Faso as early as 2024.13 This would be an experiment without any safeguards: mechanisms that effectively control a gene drive in nature exist only in theory so far. According to the current state of science, the outcome of the experiment would no longer be controllable by humans. All manipulations of this kind on animals, plants and entire ecosystems would be irreversible.
For several years, release experiments with genetically engineered insects have been taking place in the environment for research purposes. For example: since 2011, the company Oxitec in Brazil has been making repeated releases of genetically modified mosquitoes of the species Aedes aegypti. Their genetic modification was intended to render the offspring of the mosquitoes unable to reproduce.14 The goal of these releases was to significantly decimate the tropical disease-carrying mosquito population. Whether the goal was achieved is debatable.15 In any case, none of the past releases involved insects that inherit gene drives.
But what is the difference between genetically modified organisms (GMOs) and genetically modified organisms that inherit a gene drive, making them a gene drive organism (GDO)?
Goals of Gene Drive Organisms
The new dimension of genetically modifying wild populations with gene drives is in stark contrast to the previous goals, strategies, and possibilities of genetic engineering.
Until now, genetically modified organisms were either not expected to produce viable offspring, were not expected to survive long in the wild, or were prevented from mating with wild conspecifics. Thus, the use of GMOs should remain limited in space or time outside of their point of origin in the laboratory. These genetically modified organisms should not survive in the wild any more than their modified genes should.
The gene drive approach breaks radically with these considerations. In contrast to conventional GMOs, genetically modified organisms that inherit gene drives aim to spread genes synthesized in the laboratory into wild populations or to eliminate natural genes. And they do so even if this harms the species or offers it no survival advantage. This is why these genes would not prevail on the basis of natural selection.
Gene drives shift the locus of genetic modification from the genetic engineering laboratory to the wild: In the case of CRISPR/Cas9-based gene drives, the genetic engineering mechanism (CRISPR/Cas9) copies itself into the genome of wild offspring every time a GDO reproduces – over generations. The ‘forced’ inheritance of even harmful genes triggered by the gene drive triggers a theoretically unstoppable “mutagenic chain reaction”16.
CRISPR/Cas9 makes it possible
The realization of Burt’s idea of repurposing selfish genetic elements for human purposes failed for a long time due to technical hurdles. That changed in 2012, when Jennifer Doudna and Emanuelle Charpentier, now both Nobel laureates, recognized the potential of the CRISPR/Cas9 system for biotechnology.8 In bacteria, it serves as a kind of immune system to protect against viruses: The CRISPR sequence in the bacteria’s genome recognizes the invader and thus activates enzymes that attack the virus and cut up its genome.
The researchers were the first to realize that the combination of CRISPR sequences and Cas9 could be used to specifically alter the genome of many living organisms and introduce new segments into their DNA. It was the missing tool to turn Burt’s idea into reality.9 In 2015, a functional CRISPR/Cas9 gene drive in fruit flies was published for the first time.10 In the years that followed, trials in mosquitoes11 and mice12 were also successful. Researchers now suspect that almost any animal species could be manipulated with a gene drive.
How does a CRISPR based or homing gene drive work?
So-called homing gene drives based on CRISPR/Cas9 are the most common variant of synthetic gene drives. Such a gene drive consists of at least two components: the Cas9 genetic scissors and a messenger molecule. In addition, a new or modified gene can be introduced. The gene drive is first introduced into the genome of the target organism, e.g., a mouse, in the laboratory. This gene drive becomes active after fertilization of the egg cell and identifies a target sequence in the non-manipulated chromosome with the help of the messenger molecule. There, Cas9 induces a double-strand break.
Homing process
Natural repair mechanisms in the damaged cell then attempt to repair the break using a template. The gene drive on the genetically modified chromosome serves as a template: it is very likely to be copied completely and incorporated within the target sequence on the previously unmanipulated chromosome. This targeted process is called homing. In addition to the integration of the gene scissors at the target site, existing gene sequences can be switched off and/or new ones additionally inserted. This process ultimately results in all offspring inheriting a copy of the gene drive. The gene drive mechanism is re-activated with each reproduction – also in all subsequent generations – and theoretically only comes to a halt when the target sequence has disappeared from the entire population.
Sources
1 Burt A (2003). Site-specific selfish genes as tools for the control and genetic engeneering of natural populations. Proc Biol Sci 270:921
2 Werren JH (2011). Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc Natl Acad Sci U S A. 108 Suppl 2:10863
3 Stoddard BL (2011). Homing endonucleases: from microbial genetic invaders to reagents for targeted DNA modification. Structure 19:7
4 Gantz VM, Bier E (2015). The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348:442
5 O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM (1992). 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci U S A. 89(7):2699-702
6 Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell.139(7):1268-78
7 World Mosquito Website (2020). About us. Our story. Online: https://www.worldmosquitoprogram.org/en/about-us/our-story [last accessed: 22.10.2020]
8 Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012). A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 337:816
9 Esvelt KM, Smidler AL, Catteruccia F, Church GM (2014). Concerning RNA-guided gene drives for the alteration of wild populations. Elife 17:e03401
10 Gantz VM, Bier E (2015). The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348:442
11 Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A (2018). A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol 36:1062
12 Grunwald HA, Gantz VM, Poplawski G, Xu XS, Bier E, Cooper KL (2019). Super-Mendelian inheritance mediated by CRISPR-Cas9 in the female mouse germline. Nature 566:105
13 Van Woensel L, Van Steerteghem J (Scientific Foresight Unit (STOA), European Parliamentary Research ServiceScientific Foresight Unit (EPRS, 2019). The Science and ethics of gene drive technology. Case Study: Eradicating malaria. Working Breakfast; 2019 Mar 19; Europäisches Parlament, Brüssel, Belgien. p. 6. Online: https://www.europarl.europa.eu/cmsdata/161962/1-Booklet.pdf [letzter Zugriff:last accessed: 22.10.2020]
14 Oxitec website (2020). Oxitec. Brazil. Abingdon. Online: https://www.oxitec.com/brazil [last accessed Oct. 22, 2020].
15 Wallace H, Jackson A, Li Ching L, Sirinathsinghji E, Mayet M (2019). Oxitec’s failed GM mosquito releases worldwide: Forewarnings for Africa and the Target Malaria project. Online: https://www.acbio.org.za/Oxitec_failed_GM_mosquito_releases_worldwide_Forewarnings_for_Africa.pdf [letzter Zugriff:last accessed: 22.10.2020]
16 Gantz VM, Bier E (2015). The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348:442